The redesign of proteins based on an insight into their assembly and into the structural features that mediate protein-protein interfaces has allowed us to better understand and exploit these processes.
Redesign of a plant lectin allowed us to develop a system of redox-switchable agglutination for red blood cell sensing[pub 52]. Recently synthetic mimics of PTM-proteins have allowed us to develop in vivo probes of disease based on PTM-mediated protein-protein binding.[pub 85]
An interest in fundamental enzymology and binding processes of many proteins has led to the development of novel screening strategies that may be applied to many protein and all enzyme classes.[pub 59] For example, use of a powerful MS-based system has allowed us to dissect the mechanism and kinetics of glycosyltransferases[pub 66][pub 67][pub 77][pub 83] and the discovery of other synthetic carbohydrate biocatalysts.[pub 87]
This work is supported by target synthesis of libraries of substrate and ligand probes, the use of many complementary biophysical methods and structural biology. We were a member of the Basic Technology UK Glycoarray Consortium.
Our work with Dr Pierre Carcabal and Dr Emilio Cocinero is allowing us to probe the inherent conformational bias of glycans free from solvent in the gas phase.[pub 86][pub 150][pub 176][pub 182][pub 193]
This work builds on prior work with Prof. John Simons and is highlighting clear parallels with binding modes of many protein ligands and allowing the development of models of carbohydrate secondary structure.The specific inhibition of the enzymes that use sugars as their substrates (glycosidases and glycosyltransferases) provides valuable mechanistic information about their mode of action and is a therapeutic strategy for the treatment of disease.
We have developed new methods for rapidly elaborating heterocyclic scaffolds[pub 31] to create diverse libraries of glycoprocessing enzyme inhibitors.[pub 42][pub 58] From these libraries and through the development of novel affinity proteomic methods[pub 190] we can identify candidates for the treatment of diseases such as Gaucher's disease or other congenital diseases associated with carbohydrates. This work has explited novel synthetic straetgies based on stereodynamic process that allow us to construct highly substituted carbohydrate analogues that also potently inhibit such enzymes.[pub 44]
We are also interested in the design and synthesis of a novel class of transition-state analogue inhibitors of glycosyltransferases (Figure below), which are especially poorly studied enzymes.[pub 164]
We combine this synthetic work closely with an enzymological approach to the redesign of enzyme function through mutagenesis, forced evolution and structural characterization to further our understanding of such systems.
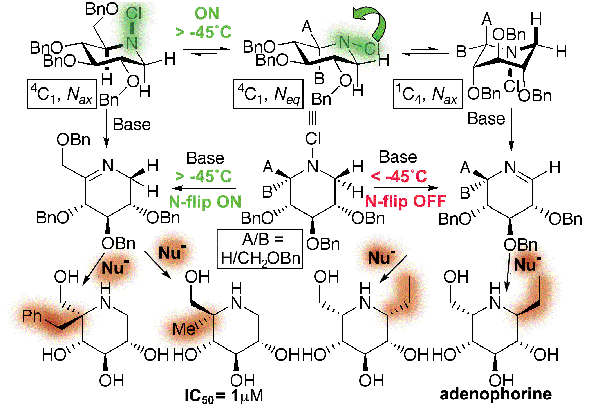
Simple use of temperature as the controlling element (Control of Lone-Pair "Flipping") allowed us to develop a novel stereodynamic synthetic approach to elaborating scaffolds of imino sugars such as the classic inhibitor DNJ.[pub 44] Moreover through appropriate understanding of the stereoselectivity of the addition of nucleophiles to cyclic sugar imines we were able to complete the first total synthesis of the naturally-occurring carbohydrate mimetic, adenophorine.
Novel Multicomponent Chemistry and the development of an Ugi-like protocol from cyclic imines has allowed the ready synthesis of focussed libraries of many hundreds of aza-sugars (imino-sugars).[pub 58]
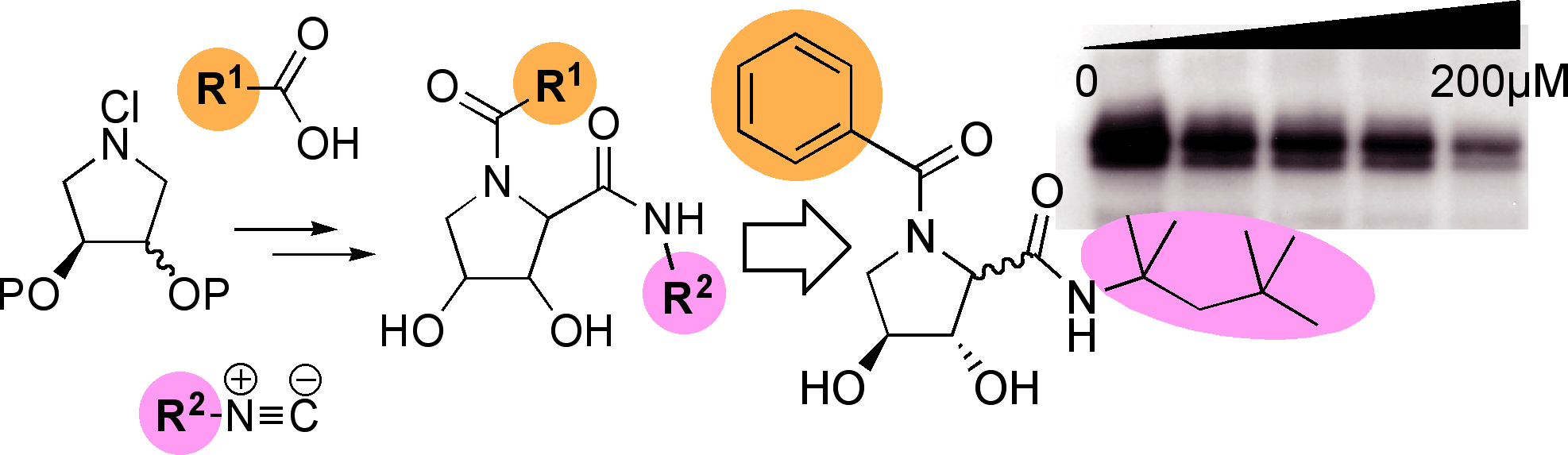
Some of these compounds are low toxicity, cell permeable agents with promising anti-viral activities. Our ongoing programme in this area is exploring their potential in chemical genetic control of intracellular glycan processing and as tools in cell biology.
- Biomolecule & Bioconjugate Construction
- Probes of Mechanism in Biology
- Exploitation in Medicine & Biotechnology
- Chemistry for Biology
- Biology for Chemistry
- Exploring, Exploiting and Designing Proteins
- Synthetic Biology
- Post-translational Modification
- New Tools for Chemical Medicine
Prof Benjamin G. Davis
University of Oxford
Chemistry Research Laboratory
Mansfield Road
Oxford, OX1 3TA, UK
Phone: + 44 (0)1865 275652
Fax: + 44 (0)1865 275674
Ben.Davis@chem.ox.ac.uk
