
Metathesis
 |
|||
Metathesis |
|||
The metathesis
reaction has recently emerged as one of the most powerful methodologies
for producing alkenes. Despite the extensive use of metathesis in organic
synthesis, there are remarkably few examples of the reaction being used
to form aromatic compounds. Thus, the group commenced investigations into
the application of the RCM reaction to provide intermediates at the correct
oxidation state to prepare fully aromatic compounds. |
||
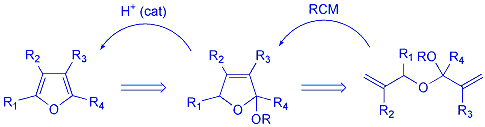
Initials
studies focused on the synthesis of furans, and we planned to utilise
the RCM reaction to form a dihydrofuran unit that is equipped with a leaving
group (OR). This intermediate could subsequently undergo an acid catalysed
elimination to furnish the corresponding furan. |
||
 |
||
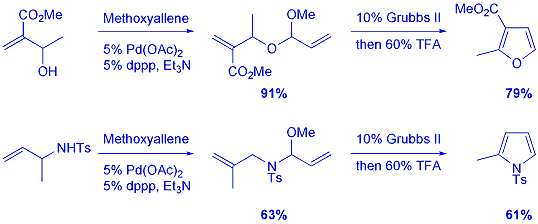
A range
of functionalised allylic alcohols were prepared, which could be transformed
into unsymmetrical mixed acetals using a palladium (II) catalysed reaction
with methoxyallene. Following successful synthesis of these precursors,
the RCM and acid catalysed aromatisation proceeded smoothly to provide
the corresponding furans. Using this methodology a range of mono- and
disubstituted furans were generated. The same sequence could be applied
to the synthesis of pyrroles by use of an allylic amine, providing a route
to 2- and 3-substituted derivatives. |
||
 |
||
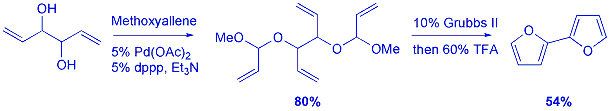
The strategy
was extended to the synthesis of linked biaryl compounds using a double
coupling, RCM and aromatisation protocol. This approach provides a rapid
and high yielding route to polyaryl compounds. |
||
 |
||
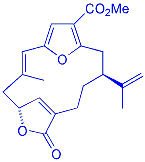
The synthesis
of deoxypukalide, a compound obtained through deoxygenation of the natural
product pukalide, is currently in progress within the group. The route
employs the RCM/aromatisation protocol as the key step to construct the
furan unit embedded within the macrocyclic product. |
||
 |
||
Deoxypukalide |