Reading Research Papers - Exercise 1
The following is extracted from a recent research article concerning membrane transport of vitamin C.
Read the article and then attempt to do the following:
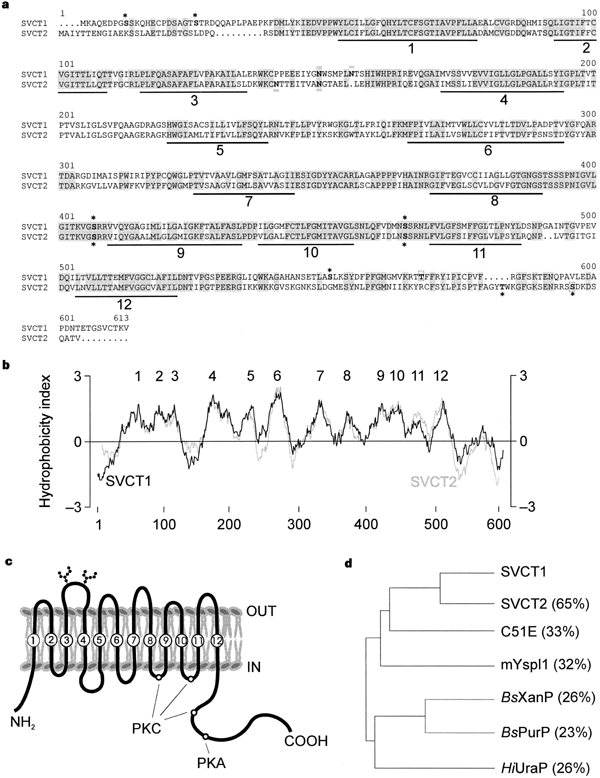
We isolated a 2,472-base-pair complementary DNA coding for a 604-amino-acid protein, SVCT1 (sodium-dependent vitamin C transporter 1), by screening a rat kidney cDNA library for Na+-dependent L-[14C]ascorbic acid transport activity in RNA-injected Xenopus oocytes (Fig. 1). Subsequent polymerase chain reaction (PCR)-based homology screening yielded a related cDNA (of 6.5 kilobases (kb), from rat brain) coding for a 592-amino-acid protein, SVCT2, with 65% amino-acid identity to SVCT1. SVCT1 and SVCT2 have similar hydropathy profiles, each predicting 12 putative membrane-spanning domains. SVCT2 is 84% identical (at the amino-acid level) to a predicted partial polypeptide encoded by the human KIAA0238 gene8; therefore, KIAA0238 may be the species homologue of rat SVCT1. SVCT1 is 59% identical to KIAA0238 and 32% identical to a mouse yolk-sac permease-like molecule (mYspl1)9 of unknown function. SVCT1 and SVCT2 shareweak homology with putative permeases of Caenorhabditis elegans and Arabidopsis thaliana , and with a bacterial purine/pyrimidine permease10. We found no significant sequence homology between SVCT and any other known family of mammalian membrane transporters11; hence SVCT1 and SVCT2 constitute a novel mammalian transporter family with a potential evolutionary link to purine/pyrimidine transporters found in prokaryotes and eukaryotes.
![]()

![]()
Figure 1 Sequence and putative membrane topology of SVCT1 and SVCT2. a, Amino-acid sequence alignment of SVCT1 with SVCT2. Putative membrane-spanning regions are underlined and numbered 1–12. Conserved residues are highlighted by shading. Potential sites for N -linked glycosylation (Asn 145, 151 for SVCT1 and Asn 132, 140 for SVCT2) (double dash), protein kinase C phosphorylation (Ser 9, 22, 403, 461, 547 for SVCT1 and Ser 397, 455, 584, Thr 571 for SVCT2) (asterisk) and cAMP-dependent phosphorylation (Thr 564 for SVCT1 and Ser 584 for SVCT2) (box). b, Kyte–Doolittle hydrophobicity plots of SVCT1 (black) and SVCT2 (grey) were generated using a 21-amino-acid window, with numbered putative membrane-spanning domains. c, Structural model of SVCT1 based on its hydropathy profile. d, SVCT superfamily phylogenic tree, with related amino-acid sequences aligned against SVCT1: C51E, Caenorhabditis elegans C51E3.6 (GenBank accession number Z78410); mYspl1, mouse yolk sac permease-like protein (U25739); Bs XanP, Bacillus subtilis xanthine permease (P42086); Bs PurP, B. subtilis urine permease (Z99120); HiUraP , Haemophilus influenzae uracil permease (P45117).
We investigated the functional characteristics of the SVCT protein family by expressing SVCT1 and SVCT2 in Xenopus oocytes and applying radiotracer and voltage-clamp techniques. Our data indicate that SVCT1 and SVCT2 each mediate saturable, Na+-dependent L-ascorbic acid transport in a rheogenic manner. L-[14C]ascorbic acid uptake (in 100 mM NaCl) followed Michaelis–Menten-type saturation kinetics with high apparent affinity for L-ascorbic acid (see Table 1 in Supplementary Information). Rates of uptake were not affected by the replacement of chloride with gluconate (data not shown), indicating that neither isoform was Cl--dependent.
In the presence of Na+,
L-ascorbic acid evoked reversible inwardcurrents of up to -100 nA in
oocytes expressing SVCT1, and up to -10 nA in oocytes expressing SVCT2 (Fig.
2). Evoked currents in control oocytes were -1 ![]() 0.5 nA
(s.d.) at 500 µM L-ascorbic
acid. Transporter-associated currents were investigated in detail for SVCT1 and,
where possible, our findings were supported by data from radiotracer experiments
for SVCT1 and SVCT2. In oocytes expressing SVCT1, the currents evoked by
L-ascorbic
acid(atsaturating levels) showed a curvilinear dependence on membrane potential
(V m) (Fig.
2b), saturating with hyperpolarization (-70 mV). The current/voltage
relationship was roughly linear between -50 and +30 mV (tending towards a
zero-current asymptote at positive V m), with no reversal of
the currents observed up to +50 mV. At -50 mV, K 0.5Asc
(the L-ascorbic acid
concentration at which currents were half-maximal) was 30 µM (Fig.
2c) with a Hill coefficient (n H) of
0.5 nA
(s.d.) at 500 µM L-ascorbic
acid. Transporter-associated currents were investigated in detail for SVCT1 and,
where possible, our findings were supported by data from radiotracer experiments
for SVCT1 and SVCT2. In oocytes expressing SVCT1, the currents evoked by
L-ascorbic
acid(atsaturating levels) showed a curvilinear dependence on membrane potential
(V m) (Fig.
2b), saturating with hyperpolarization (-70 mV). The current/voltage
relationship was roughly linear between -50 and +30 mV (tending towards a
zero-current asymptote at positive V m), with no reversal of
the currents observed up to +50 mV. At -50 mV, K 0.5Asc
(the L-ascorbic acid
concentration at which currents were half-maximal) was 30 µM (Fig.
2c) with a Hill coefficient (n H) of ![]() 1.
The n H for Na+ was
1.
The n H for Na+ was ![]() 2
(Fig.
2d). K 0.5Na (at 200 µM
L-ascorbic
acid) was 40 mM at -50 mV; K 0.5Na was not
significantly different at hyperpolarized V m, but rose at
depolarized V m (data not shown). These data indicate that two
Na+ ions bind to SVCT1 and that binding is voltage-sensitive.
SVCT1-mediated L-ascorbic acid
transport is driven by the electrochemical gradient for Na+ because,
at any given V m, the current evoked by 200 µM
L-ascorbic acid was greatest at higher Na+ concentrations (Fig.
2e). The evoked currents obtained at 100 mM Na+ saturated at a
less negative V m and with a larger maximal current than those
obtained at 50 and 20 mM Na+. Following step-changes in voltage (not
shown), SVCT1 exhibited pre-steady-state currents (decaying with time constants
of 10–40 ms). These pre-steady-state currents were sensitive to changes in
extracellular Na+ concentration and (in analogy to other ion-coupled
transporters12,13)
are due in part to binding/dissociation of the driving ion (in this case Na+)
to the transporter. Switching from 0 to 100 mM Na+ in the absence of
L-ascorbic
acid caused a small inward current in control occytes (Fig.2a,
g), but a much larger current in oocytes expressing either SVCT1 or SVCT2.
Addition of L-ascorbic acid in the
presence of Na+ evoked still larger inward currents. These
observations indicate that SVCT1 and SVCT2 can additionally exhibit a Na+
leak (uniport) current (similar to the leak pathways in other ion-coupled
transporters14-16)
that is as much as half the magnitude of the current associated with Na+/L-ascorbic acid co-transport (Fig.
2g).
2
(Fig.
2d). K 0.5Na (at 200 µM
L-ascorbic
acid) was 40 mM at -50 mV; K 0.5Na was not
significantly different at hyperpolarized V m, but rose at
depolarized V m (data not shown). These data indicate that two
Na+ ions bind to SVCT1 and that binding is voltage-sensitive.
SVCT1-mediated L-ascorbic acid
transport is driven by the electrochemical gradient for Na+ because,
at any given V m, the current evoked by 200 µM
L-ascorbic acid was greatest at higher Na+ concentrations (Fig.
2e). The evoked currents obtained at 100 mM Na+ saturated at a
less negative V m and with a larger maximal current than those
obtained at 50 and 20 mM Na+. Following step-changes in voltage (not
shown), SVCT1 exhibited pre-steady-state currents (decaying with time constants
of 10–40 ms). These pre-steady-state currents were sensitive to changes in
extracellular Na+ concentration and (in analogy to other ion-coupled
transporters12,13)
are due in part to binding/dissociation of the driving ion (in this case Na+)
to the transporter. Switching from 0 to 100 mM Na+ in the absence of
L-ascorbic
acid caused a small inward current in control occytes (Fig.2a,
g), but a much larger current in oocytes expressing either SVCT1 or SVCT2.
Addition of L-ascorbic acid in the
presence of Na+ evoked still larger inward currents. These
observations indicate that SVCT1 and SVCT2 can additionally exhibit a Na+
leak (uniport) current (similar to the leak pathways in other ion-coupled
transporters14-16)
that is as much as half the magnitude of the current associated with Na+/L-ascorbic acid co-transport (Fig.
2g).
![]()
![]() Figure
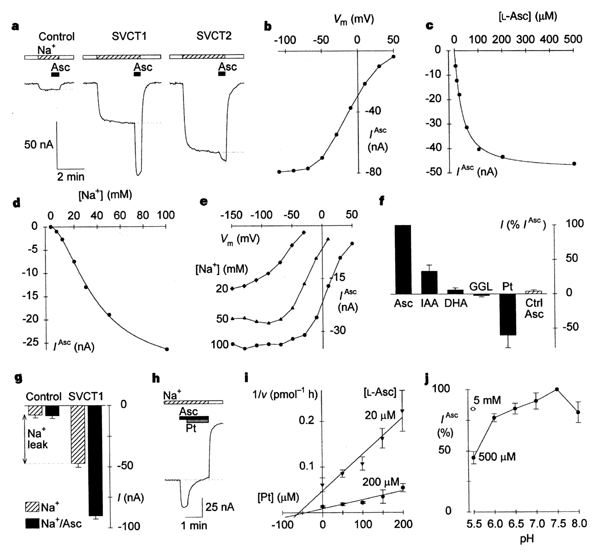
2 Functional characterization of SVCT expressed in Xenopus oocytes.
a, Typical current recordings from a control oocyte, and oocytes
expressing SVCT1 and SVCT2, all from a single batch of oocytes. The approximate
baseline current in standard Na+ medium at pH 7.5 (hatched boxes) is
indicated by the dotted line. 500 µM L-ascorbic
acid (Asc) was superfused (in Na+ medium) for the periods indicated
by the black boxes, and substrates were always washed out in choline medium
(blank boxes). b, Current/voltage (I/V ) relationship for SVCT1 at
500 µM (saturating) L-ascorbic acid (at 100 mM Na+). c,
L-Ascorbic acid saturation kinetics for
SVCT1 at 100 mM Na+ and at -50 mV (K0.5Asc
= 29
Figure
2 Functional characterization of SVCT expressed in Xenopus oocytes.
a, Typical current recordings from a control oocyte, and oocytes
expressing SVCT1 and SVCT2, all from a single batch of oocytes. The approximate
baseline current in standard Na+ medium at pH 7.5 (hatched boxes) is
indicated by the dotted line. 500 µM L-ascorbic
acid (Asc) was superfused (in Na+ medium) for the periods indicated
by the black boxes, and substrates were always washed out in choline medium
(blank boxes). b, Current/voltage (I/V ) relationship for SVCT1 at
500 µM (saturating) L-ascorbic acid (at 100 mM Na+). c,
L-Ascorbic acid saturation kinetics for
SVCT1 at 100 mM Na+ and at -50 mV (K0.5Asc
= 29 ![]() 3 µM;
ImaxAsc = -49
3 µM;
ImaxAsc = -49 ![]() 1 nA;
nH = 1.1
1 nA;
nH = 1.1 ![]() 0.1).
d, Na+ saturation kinetics of the currents evoked by 200 µM
L-ascorbic acid for SVCT1 at -50 mV (n
H for Na+ = 1.7
0.1).
d, Na+ saturation kinetics of the currents evoked by 200 µM
L-ascorbic acid for SVCT1 at -50 mV (n
H for Na+ = 1.7 ![]() 0.1;
K0.5Na = 38
0.1;
K0.5Na = 38 ![]() 2 mM;
ImaxNa = -31
2 mM;
ImaxNa = -31 ![]() 1 nA).
e, The effect of Na+ on the I/V relationship for SVCT1
(at200 µM L-ascorbic acid). f,
Substrate selectivity of SVCT1. Currents (mean
1 nA).
e, The effect of Na+ on the I/V relationship for SVCT1
(at200 µM L-ascorbic acid). f,
Substrate selectivity of SVCT1. Currents (mean ![]() s.e.m.,
n = 7–12 oocytes) evoked by test substrates applied at 500 µM (at -50 mV)
were normalized to the L-ascorbic
acid-evoked current (-25
s.e.m.,
n = 7–12 oocytes) evoked by test substrates applied at 500 µM (at -50 mV)
were normalized to the L-ascorbic
acid-evoked current (-25 ![]() 4 nA,
n = 12); IAA, D-isoascorbic acid;
DHA, dehydroascorbic acid; GGL, L-gulono-
4 nA,
n = 12); IAA, D-isoascorbic acid;
DHA, dehydroascorbic acid; GGL, L-gulono-![]() -lactone;
Pt, phloretin; Ctrl Asc, L-ascorbic
acid-evoked current in control oocytes. g, Comparison of currents due to
Na+ leak (uniport) and Na+/L-ascorbic
acid (Na+/Asc) co-transport pathways in SVCT1. Currents resulting
from the addition of either 100 mM Na+ alone (hatched bars) or 100 mM
Na+ plus 500 µM L-ascorbic acid
(Na+/Asc) (solid bars) were compared in control oocytes and oocytes
expressing SVCT1 (mean
-lactone;
Pt, phloretin; Ctrl Asc, L-ascorbic
acid-evoked current in control oocytes. g, Comparison of currents due to
Na+ leak (uniport) and Na+/L-ascorbic
acid (Na+/Asc) co-transport pathways in SVCT1. Currents resulting
from the addition of either 100 mM Na+ alone (hatched bars) or 100 mM
Na+ plus 500 µM L-ascorbic acid
(Na+/Asc) (solid bars) were compared in control oocytes and oocytes
expressing SVCT1 (mean ![]() s.e.m.,
n = 5). Zero represents the current in Na+-free medium. h,
Phloretin (Pt) inhibition of the L-ascorbic
acid-evoked current in SVCT1. An oocyte expressing SVCT1 was superfused (at
-50 mV) in standard Na+ medium (hatched box) before the addition of
200 µM L-ascorbic acid (black box) and
100 µM phloretin (grey box), before washing out with choline medium (blank box);
the approximate baseline current in Na+ is indicated by the dotted
line. i, Dixon analysis of phloretin inhibition.
L-[14C] ascorbic acid uptake,
v (mean
s.e.m.,
n = 5). Zero represents the current in Na+-free medium. h,
Phloretin (Pt) inhibition of the L-ascorbic
acid-evoked current in SVCT1. An oocyte expressing SVCT1 was superfused (at
-50 mV) in standard Na+ medium (hatched box) before the addition of
200 µM L-ascorbic acid (black box) and
100 µM phloretin (grey box), before washing out with choline medium (blank box);
the approximate baseline current in Na+ is indicated by the dotted
line. i, Dixon analysis of phloretin inhibition.
L-[14C] ascorbic acid uptake,
v (mean ![]() s.e.m.
from 6–10 oocytes, 60 min), was determined at 20 µM or 200 µM
L-ascorbic acid in the presence of
0–200 µM phloretin. From linear regression, thereciprocal plots (20 µM, r2
= 0.90; 200 µM, r2 = 0.95) intersected at
s.e.m.
from 6–10 oocytes, 60 min), was determined at 20 µM or 200 µM
L-ascorbic acid in the presence of
0–200 µM phloretin. From linear regression, thereciprocal plots (20 µM, r2
= 0.90; 200 µM, r2 = 0.95) intersected at ![]() -65 µM
(-K iPt). j, pH dependence of
L-ascorbic acid-evoked currents in SVCT1.
Currents evoked by 500 µM L-ascorbic acid
(filled circles) at extracellular pH 5.5–8.0 were normalized to the current at
pH 7.5 (-28
-65 µM
(-K iPt). j, pH dependence of
L-ascorbic acid-evoked currents in SVCT1.
Currents evoked by 500 µM L-ascorbic acid
(filled circles) at extracellular pH 5.5–8.0 were normalized to the current at
pH 7.5 (-28 ![]() 2 nA,
mean
2 nA,
mean ![]() s.e.m.,
n = 6). The current evoked by 5 mM L-ascorbic
acid at pH 5.5 is also shown (open circle).
s.e.m.,
n = 6). The current evoked by 5 mM L-ascorbic
acid at pH 5.5 is also shown (open circle).
SVCT1 was highly selective for
L-ascorbic acid, which evoked much larger currents than did
D-isoascorbic acid (![]() 30%
of that forL-ascorbic acid) or dehydroascorbic acid (
30%
of that forL-ascorbic acid) or dehydroascorbic acid (![]() 5%)
(Fig.
2f). (Dehydroascorbic acid did not evoke a current in control oocytes.)
D-glucose,
uracil (not shown) and intermediates of vitamin C metabolism (such as
L-gulono-
5%)
(Fig.
2f). (Dehydroascorbic acid did not evoke a current in control oocytes.)
D-glucose,
uracil (not shown) and intermediates of vitamin C metabolism (such as
L-gulono-![]() -lactone)
were excluded. Several test compounds, including aspirin (acetylsalicylic acid),
xanthine, sulfinpyrazone and phlorizin, each evoked tiny outward currents in
oocytes expressing SVCT1 and also slightly inhibited SVCT1- or SVCT2-mediated
L-[14C]ascorbic acid uptake (data not shown); that is, they
exhibited characteristics of weak blockers. Phloretin evoked a sizeable outward
current in oocytes expressing SVCT1 (Fig.
2f). We attribute this effect to phloretin blocking the Na+ leak
current. In addition, phloretin blocked the inward current evoked by
L-ascorbic
acid (Fig.
2h) and inhibited L-[14C]ascorbic
acid uptake by SVCT1 or SVCT2. Dixon analysis of
L-[14C]ascorbic
acid uptake for SVCT1 (Fig.
2i) revealed that phloretin was a non-competitive inhibitor17
with Ki
-lactone)
were excluded. Several test compounds, including aspirin (acetylsalicylic acid),
xanthine, sulfinpyrazone and phlorizin, each evoked tiny outward currents in
oocytes expressing SVCT1 and also slightly inhibited SVCT1- or SVCT2-mediated
L-[14C]ascorbic acid uptake (data not shown); that is, they
exhibited characteristics of weak blockers. Phloretin evoked a sizeable outward
current in oocytes expressing SVCT1 (Fig.
2f). We attribute this effect to phloretin blocking the Na+ leak
current. In addition, phloretin blocked the inward current evoked by
L-ascorbic
acid (Fig.
2h) and inhibited L-[14C]ascorbic
acid uptake by SVCT1 or SVCT2. Dixon analysis of
L-[14C]ascorbic
acid uptake for SVCT1 (Fig.
2i) revealed that phloretin was a non-competitive inhibitor17
with Ki ![]() 65 µM.
Phloretin also inhibited the Na+ leak current with an apparent Ki
65 µM.
Phloretin also inhibited the Na+ leak current with an apparent Ki ![]() 100 µM
(data not shown). The similarity of these values for the effects of phloretin on
both the co-transport and uniport modes of the transport cycle indicates that
phloretin may interact with SVCT1 at a single locus on the protein. The evoked
currents in SVCT1 were sensitive to changes in extracellular pH (Fig.
2j). Those at pH 5.5 were 50% smaller than those at pH 7.5, but were partly
restored by the addition of excess
L-ascorbic acid (5 mM). SVCT2 showed a similar pattern of pH sensitivity
in radiotracer experiments (not shown). This pH sensitivity is probably a result
of reduced binding affinities for
L-ascorbic acid, rather than less available
L-ascorbic acid in the deprotonated (1-) form, as more than 95% is in the
1- form at pH 5.5 (pKa1 = 4.2).
100 µM
(data not shown). The similarity of these values for the effects of phloretin on
both the co-transport and uniport modes of the transport cycle indicates that
phloretin may interact with SVCT1 at a single locus on the protein. The evoked
currents in SVCT1 were sensitive to changes in extracellular pH (Fig.
2j). Those at pH 5.5 were 50% smaller than those at pH 7.5, but were partly
restored by the addition of excess
L-ascorbic acid (5 mM). SVCT2 showed a similar pattern of pH sensitivity
in radiotracer experiments (not shown). This pH sensitivity is probably a result
of reduced binding affinities for
L-ascorbic acid, rather than less available
L-ascorbic acid in the deprotonated (1-) form, as more than 95% is in the
1- form at pH 5.5 (pKa1 = 4.2).
The function of SVCT2 was primarily investigated by radio tracer uptake studies because the currents were relatively small. The studies did not reveal any functional differences between the two SVCT isoforms. The activities of SVCT1 and SVCT2 expressed in oocytes were consistent with those of several mammalian tissues (in vesicles, isolated tissues or cultured cell lines)18-22 with regard to the following characteristics: high apparent affinity for L-ascorbic acid (K 0.5Asc of 10–100 µM); a preference for L-ascorbic acid over D-isoascorbic acid, which is preferred over dehydroascorbic acid; and Na+ dependence.
We found striking differences between SVCT1
and SVCT2 in terms of tissue distribution. Northern blot analysis using a probe
for SVCT1 revealed intense bands at ![]() 2.5 kb
and
2.5 kb
and ![]() 4.0 kb
in kidney, intestine and liver, whereas probing for SVCT2 RNA resulted in a
weaker signal at
4.0 kb
in kidney, intestine and liver, whereas probing for SVCT2 RNA resulted in a
weaker signal at ![]() 6.5 kb
for those tissues (see Fig. 5 in
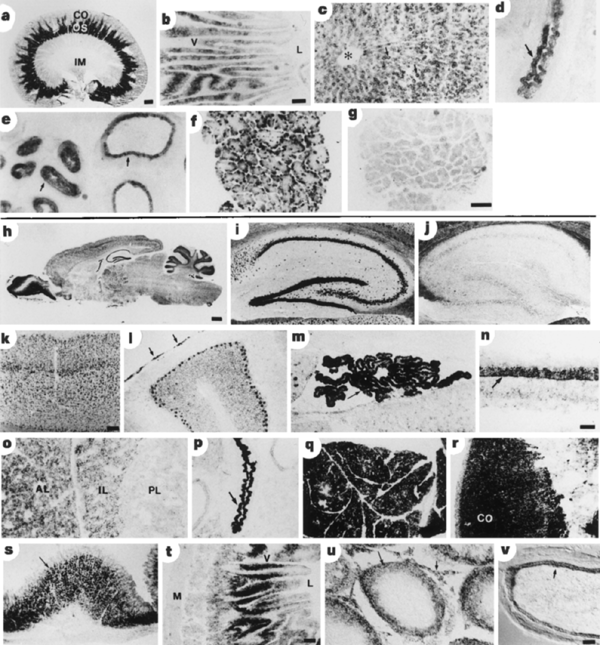
Supplementary Information). Using in situ hybridization (Fig.
3), SVCT1 was localized to the straight segment (S3) of the proximal tubule
in the kidney, consistent with studies of rat kidney
L-ascorbic acid transport in vitro18,19.
SVCT1 and SVCT2 messenger RNAs were present in enterocytes in the small
intestine. In liver, SVCT1 but not SVCT2 was detected in hepatocytes. Both
isoforms were detected in epithelial cells of the bronchiole and epididymis.
6.5 kb
for those tissues (see Fig. 5 in
Supplementary Information). Using in situ hybridization (Fig.
3), SVCT1 was localized to the straight segment (S3) of the proximal tubule
in the kidney, consistent with studies of rat kidney
L-ascorbic acid transport in vitro18,19.
SVCT1 and SVCT2 messenger RNAs were present in enterocytes in the small
intestine. In liver, SVCT1 but not SVCT2 was detected in hepatocytes. Both
isoforms were detected in epithelial cells of the bronchiole and epididymis.
![]()
![]()
Figure 3 Localization of SVCT1 and SVCT2 mRNA in rat tissues detected by insitu hybridization. Bright-field micrographs of cryosections hybridized to digoxigenin-labelled antisense cRNA probes of SVCT1 (a–f) and SVCT2 (h, i, k–v). Those hybridized to sense cRNA probes for SVCT1 (g) and SVCT2 (j) revealed no significant signal. a, Kidney (CO, cortex; OS, outer stripe of the medulla; IM, inner medulla). b, Duodenum (V, villi; L, lumen). c, Liver (asterisk, central vein; arrows indicate hepatocytes). d, Lung (arrow, bronchiolar epithelium). e, Epididymis (arrows, tubules). f, Lacrimal gland. g, Lacrimal gland (sense). h, Brain. i, Hippocampus. j, Hippocampus (sense). k, Cerebral cortex. l, Cerebellum (arrows, labelled meningeal layers). m, Choroid plexus (arrow). n, Retina (arrow, inner nuclear layer). o, Pituitary gland (AL, anterior lobe; IL, intermediate lobe; PL, posterior lobe). p, Lung (arrow, bronchiolar epithelium). q, Pancreas. r, Adrenal gland (CO, cortex). s, Stomach (arrow, gastric glands). t, Duodenum (M, muscle layer; V, villi; L, lumen). u, Testis (long arrow, spermatocytes; short arrow, interstitial cells). v, Epididymis (arrow, tubular epithelium). Scale bars: a, h, 1 mm; b, 100 µm; c–g, 100 µm (shown in g); l, p–t, 100 µm (shown in t); i–k, 200 µm (shown in k); m, o, u, v, 50 µm (shown in v); n, 50 µm.
SVCT2 was abundantly expressed in a range of
neural, neuroendocrine, exocrine and endothelial tissues as well as in
osteoblasts. Northern blot analysis gave a strong SVCT2 signal for brain at ![]() 6.5 kb.
The mRNA was localized to neurons throughout the central nervous system. The
distribution closely matched the sites of rapid neuronal accumulation observed
in mouse brain following intravenous injection of
L-[14C]ascorbic
acid23
and is consistent with the view that neurons take up
L-ascorbic acid (released by astroglia). SVCT2 was also detected in the
meninges and choroid plexus, indicating that SVCT2 may facilitate entry of
L-ascorbic acid into the cerebrospinal fluid compartments. In the retina,
SVCT2 labelling was observed exclusively in the inner nuclear layer (the site of
bipolar, amacrine and horizontal-cell bodies).
6.5 kb.
The mRNA was localized to neurons throughout the central nervous system. The
distribution closely matched the sites of rapid neuronal accumulation observed
in mouse brain following intravenous injection of
L-[14C]ascorbic
acid23
and is consistent with the view that neurons take up
L-ascorbic acid (released by astroglia). SVCT2 was also detected in the
meninges and choroid plexus, indicating that SVCT2 may facilitate entry of
L-ascorbic acid into the cerebrospinal fluid compartments. In the retina,
SVCT2 labelling was observed exclusively in the inner nuclear layer (the site of
bipolar, amacrine and horizontal-cell bodies).
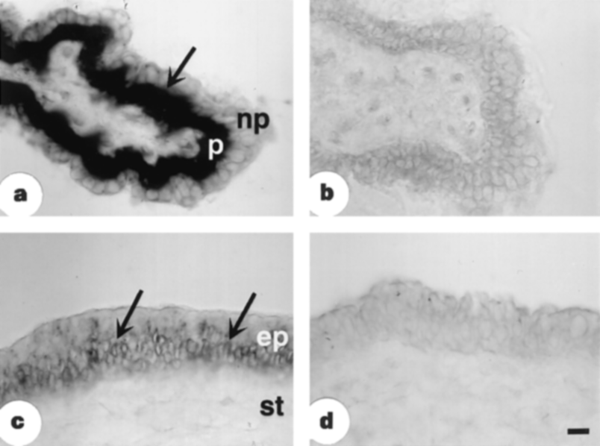
L-ascorbic acid is believed to be crucial in protecting components of the eye (such as the lens and cornea) from radiation-induced damage24. As the levels of L-ascorbic acid in the aqueous humour (anterior chamber) in diurnal mammals can be around 20-fold greater than in nocturnal mammals25, we investigated whether the distribution of SVCT isoforms in the eye differed between the rat (nocturnal) and the rabbit (diurnal–nocturnal). In the rat, neither SVCT1 nor SVCT2 was detected in the ciliary body (which secretes aqueous humour). However, in the albino rabbit, SVCT2 was abundantly expressed in the pigmented epithelium of the ciliary body, and moderately expressed in the deeper layers of the corneal epithelium (Fig. 4). This SVCT2 distribution was consistent with the known sites of L-ascorbic acid concentration in the rabbit eye26, and the characteristics of SVCT2 reflected those of a Na+-dependent L-ascorbic acid transport activity expressed in bovine pigmented ciliary epithelial cells20. In the rat, SVCT1 (Fig. 3) and SVCT2 (not shown) were each detected in exocrine cells of the lacrimal gland. There, the supply of L-ascorbic acid to the tear fluid may provide sufficient antioxidant protection in nocturnal species.
![]()
![]()
Figure 4 Localization of SVCT2 mRNA in the albino rabbit eye detected by in situ hybridization. Bright-field micrographs of cryosections hybridized to digoxigenin-labelled antisense cRNA probe showing: a, the pigmented (p) and nonpigmented (np) cell layers in ciliary body and ciliary processes; b, sense control for ciliary process; c, Deep layers of the corneal epithelium (arrows); ep, epithelium; st, stroma of the cornea; d, Sense control for corneal epithelium. Scale bar, 20 µm.
SVCT2 was expressed in several components of
the endocrine system, including the anterior pituitary, in which
L–ascorbic acid is important in ![]() -amidiation
of peptide hormones. Labelling was also observed in the intermediate lobe but
not in the posterior lobe. SVCT2 was abundantly expressed throughout the
pancreas and in the adrenal cortex. In the adrenal gland,
L-ascorbic acid is
critical for the copper-associated dopamine-
-amidiation
of peptide hormones. Labelling was also observed in the intermediate lobe but
not in the posterior lobe. SVCT2 was abundantly expressed throughout the
pancreas and in the adrenal cortex. In the adrenal gland,
L-ascorbic acid is
critical for the copper-associated dopamine-![]() -hydroxylase
involved in noradrenaline synthesis2.
SVCT2 was detected in gastric glands, extending from the base to the isthmus.
Autoradiographic studies in mouse demonstrated rapid accumulation of
L-ascorbic acid into the gastric mucosa following intravenous injection23.
These observations implicate SVCT2 in the basolateral uptake of
L-ascorbic acid, for secretion by the gastric gland, aiding dietary
absorption of iron. An intense SVCT2-related signal was obtained in northern
blot analysis of poly(A)+ RNA from the murine osteoblast MC3T3-E1
cell line22.
SVCT2-mediated L-ascorbic
acid transport into osteoblasts may account for the adequate supply of the
vitamin to maintain Fe2+ required by hydroxylases involved in
collagen synthesis2.
Among immune system organs, SVCT2 was detected by northern blot in the spleen
and thymus. In testis, SVCT2 was expressed in interstitial cells and
spermatocytes.
-hydroxylase
involved in noradrenaline synthesis2.
SVCT2 was detected in gastric glands, extending from the base to the isthmus.
Autoradiographic studies in mouse demonstrated rapid accumulation of
L-ascorbic acid into the gastric mucosa following intravenous injection23.
These observations implicate SVCT2 in the basolateral uptake of
L-ascorbic acid, for secretion by the gastric gland, aiding dietary
absorption of iron. An intense SVCT2-related signal was obtained in northern
blot analysis of poly(A)+ RNA from the murine osteoblast MC3T3-E1
cell line22.
SVCT2-mediated L-ascorbic
acid transport into osteoblasts may account for the adequate supply of the
vitamin to maintain Fe2+ required by hydroxylases involved in
collagen synthesis2.
Among immune system organs, SVCT2 was detected by northern blot in the spleen
and thymus. In testis, SVCT2 was expressed in interstitial cells and
spermatocytes.
To assess the relative contributions to L-ascorbic acid transport of SVCT1 and SVCT2 or possible additional transporters, we performed hybrid depletion of poly(A)+ RNA isolated from several rat tissues and monitored transport activity in oocytes (see Fig. 6 in Supplementary Information). L-[14C]ascorbic acid uptake was threefold higher in oocytes injected with intestinal RNA than in control oocytes. The induced activity was Na+-dependent (not shown) and abolished by hybridization with antisense oligonucleotide against SVCT1, indicating that SVCT1 is the predominant L-ascorbic acid transport system in the small intestine. Similar results were obtained for kidney, and data for adrenal gland indicate that expression of SVCT2 and SVCT1 can account for the increased uptake in RNA-injected oocytes. SVCT1 and SVCT2 therefore seem to be the predominant L-ascorbic acid transport systems (at least for K 0.5Asc of similar order) in the tissues tested.
Our data show that SVCT1 and SVCT2 play central roles in the absorption and accumulation of vitamin C in many tissues. SVCT1 is largely confined to bulk-transporting epithelia, in which it may serve whole-body homeostasis and metabolic requirements for the vitamin, whereas SVCT2 may account for the widespread tissue-specific uptake of L-ascorbic acid required for an array of biological functions, including enzymatic reactions and antioxidation. To serve these roles, we speculate that SVCT1 is located on the apical membrane, whereas SVCT2 could be directed to the basolateral membrane in endocrine or exocrine tissues. However, such polarization must be verified by immunocytochemistry. Despite their discrete distribution, SVCT1 and SVCT2 displayed very similar functional characteristics when expressed in oocytes. Molecular cloning of SVCT1 and SVCT2 now enables us to examine the possibility of differential regulation of these two transporters and how they may adapt to oxidative stress in pathological conditions.
Note added in proof . Related sequences have recently been reported but their function remains unknown (AF058319, AF058317, Biochim. Biophys. Acta 1442, 353–360, 1998).
Methods
Detailed methods are provided in the
Supplementary Information. Briefly, the SVCT1 cDNA was isolated from rat
kidney by expression cloning as described27
and the SVCT2 cDNA was subsequently isolated by PCR-based homology screening of
a rat brain cDNA library. A 1.2-kb rabbit cDNA with 96% identity to residues
96–505 of the rat SVCT2 was obtained by RT-PCR. Radiotracer and voltage-clamp
experiments were performed (at 22 °C) in Xenopus oocytes (isolated and
maintained as described28)
2–7 days after injection with ![]() 25 ng
of SVCT1 or SVCT2 cRNA synthesized in vitro . Radiotracer uptake was
determined by incubating 6–10 oocytes for 30 or 60 min in standard 100 mM Na+
or Na+-free media with 10–600 µM
L-[1-14C]ascorbic
acid. Steady-state currents measured using a two-microelectrode voltage-clamp
were fitted to equation 11.3 of
ref. 28. Protocols used in northern blot analysis, in situ
hybridization (with digoxigenin-labelling as described29)
and hybrid depletion studies are detailed in the
Supplementary Information.
25 ng
of SVCT1 or SVCT2 cRNA synthesized in vitro . Radiotracer uptake was
determined by incubating 6–10 oocytes for 30 or 60 min in standard 100 mM Na+
or Na+-free media with 10–600 µM
L-[1-14C]ascorbic
acid. Steady-state currents measured using a two-microelectrode voltage-clamp
were fitted to equation 11.3 of
ref. 28. Protocols used in northern blot analysis, in situ
hybridization (with digoxigenin-labelling as described29)
and hybrid depletion studies are detailed in the
Supplementary Information.
References
|
1. |
Englard, S. & Seifter, S. The biochemical functions of ascorbic acid. Annu. Rev. Nutr. 6, 365-406 (1986). | Article | PubMed | ISI | |
|
2. |
Padh, H. Vitamin C: newer insights into its biochemical functions. Nutr. Rev. 49, 65-70 (1991). | PubMed | ISI | |
|
3. |
Rose, R. C. & Bode, A. M. Biology of the free radical scavengers: an evaluation of ascorbate. FASEB J. 7, 1135-1142 (1993). |
|
4. |
Vera, J. C., Rivas, C. I. & Fischbarg, J. Mammalian facilitative hexose transporters mediate the transport of dehydroascorbic acid. Nature 364, 79-82 (1993). | PubMed | ISI | |
|
5. |
Rumsey, S. C. et al . Glucose transporter isoforms GLUT1 and GLUT3 transport dehydroascorbic acid. J. Biol. Chem. 272, 18982-18989 (1997). | Article | |
|
6. |
Agus, D. B. et al . Vitamin C crosses the blood-brain barrier in the oxidized from through the glucose transporters. J. Clin. Invest. 100, 2842-2848 (1997). |
|
7. |
Dhariwal, K. R., Hartzell, W. O. & Levine, M. Ascorbic acid and dehydroascorbic acid measurements in human plasma and serum. Am. J. Clin. Nutr. 54, 712-716 (1991). | PubMed | ISI | |
|
8. |
Nagase, T. et al . Prediction of the coding sequences of unidentified human genes. VI. The coding sequences of 80 new genes (KIAA0201 -KIAA0280 ) deduced by analysis of cDNA clones from cell line KG-1 and brain. DNA Res. 3, 321-354 (1996). | PubMed | |
|
9. |
Guimaraes, M. J. et al . Anew approach to the study of haematopoietic development in the yolk sac and embryoid bodies. Development 121, 3335-3346 (1995). | PubMed | ISI | |
|
10. |
Diallinas, G., Gorfinkiel, L., Arst, H. N., Cecchetto, G. & Scazzocchio, C. Genetic and molecular characterization of a gene encoding a wide specificity purine permease of Aspergillus nidulans reveals a novel family of transporters conserved in prokaryotes and eukaryotes. J. Biol. Chem. 270, 8610-8622 (1995). | Article | PubMed | ISI | |
|
11. |
Wright, E. M., Loo, D. D. F., Turk, E. & Hirayama, B. A. Sodium cotransporters. Curr. Opin. Cell Biol. 8, 468-473 (1996). | Article | PubMed | |
|
12. |
Hazama, A., Loo, D. D. F. & Wright, E. M. Presteady-state currents of the rabbit Na+/glucose cotransporter (SGLT1). J. Membr. Biol. 155, 175-186 (1997). | Article | PubMed | |
|
13. |
Mackenzie, B., Loo, D. D. F., Panayotova-Heiermann, M. & Wright, E. M. Biophysical characteristics of the pig kidney Na+/glucose cotransporter SGLT2 reveal a common mechanism for SGLT1 and SGLT2. J. Biol. Chem. 271, 32678-32683 (1996). | Article | PubMed | ISI | |
|
14. |
Mackenzie, B., Loo, D. D. F. & Wright, E. M. Relationships between Na+/glucose cotransporter currents and fluxes. J. Membr. Biol. 162, 101-106 (1998). | Article | PubMed | |
|
15. |
Gunshin, H. et al . Cloning and characterization of a proton-coupled mammalian metal-ion transporter. Nature 388, 482-488 (1997). | Article | PubMed | |
|
16. |
Eskandari, S. et al . Thyroid Na+/I symporter: mechanism, stoichiometry, and specificity. J. Biol. Chem. 272, 27230-27238 (1997). | Article | |
|
17. |
Segel, I. H. Biochemical Calculations2nd edn(Wiley, New York, (1976). |
|
18. |
Bowers-Komro, D. M. & McCormick, D. B. Characterization of ascorbic acid uptake by isolated rat kidney cells. J. Nutr. 121, 57-64 (1991). |
|
19. |
Toggenburger, G. et al . Na+-dependent, potential-sensitive L-ascorbate transport across brush border membrane vesicles from kidney cortex. Biochim. Biophys. Acta 646, 433-443 (1981). |
|
20. |
Helbig, H. et al . Electrogenic Na+-ascorbate cotransport in cultured bovine pigmented ciliary epithelial cells. Am. J. Physiol. 256, C44-C49 (1989). | PubMed | ISI | |
|
21. |
Rose, R. C. & Wilson, J. X. in Vitamin C in Health and Disease(eds Packer, L. & Fuchs, J.) 143-161 (Dekker, New York, (1997). |
|
22. |
Franceschi, R. T., Wilson, J. X. & Dixon, S. J. Requirement for Na+-dependent ascorbic acid transport in osteoblast function. Am. J. Physiol. 268, C1430-C1439 (1995). | PubMed | |
|
23. |
Hammarström, L. Autoradiographic studies on the distribution of C14-labelled ascorbic acid and dehydroascorbic acid. Acta Physiol. Scand. 70 (suppl.)289, 1–75 (1966). |
|
24. |
Rose, R. C. & Bode, A. M. Ocular ascorbate transport and metabolism. Comp. Biochem. Physiol. 100, 273-285 (1991). |
|
25. |
Reiss, G. R., Werness, P. G., Zollman, P. E. & Brubaker, R. F. Ascorbic acid levels in the aqueous humor of nocturnal and diurnal mammals. Arch. Ophthalmol. 104, 753-755 (1986). | PubMed | ISI | |
|
26. |
Kodama, T., Kabasawa, I., Tamura, O. & Reddy, V. N. Dynamics of ascorbate in the aqueous humor and tissues surrounding ocular chambers. Ophthalmic Res. 17, 331-337 (1985). | PubMed | ISI | |
|
27. |
Romero, M. F., Kanai, Y., Gunshin, H. & Hediger, M. A. Expression cloning using Xenopus laevis oocytes. Methods Enzymol. 296, 17-52 (1998). |
|
28. |
Mackenzie, B. in Biomembrane Transport(ed. Van Winkle, L. J.) 327-342 (Academic, San Diego, (1999). |
|
29. |
Schaeren-Wiemers, N. & Gerfin-Moser, A. Asingle protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridization using digoxigenin-labelled cRNA probes. Histochemistry 100, 431-440 (1993). |