|
Cation exchange reactions allow the preparation of metastable phases which cannot be prepared directly by high temperature synthesis routes. While most cations within extended oxide lattice are not sufficiently mobile to allow low-temperature cation exchange to occur at an appreciable rate, monovalent cations such those of the group 1 metals (Li+, Na+, K+, Rb+, Cs+) or Ag+ can be exchanged if located within robust extended lattices. A particularly useful application of this type of reaction is exchanging these diamagnetic cations for paramagnetic transition metal cations, such as Ni2+ or Co2+, as a method of introducing paramagnetic behaviour into materials.
|
Cation-exchange as a route to multiferroic materials
The preparation of materials which exhibit magnetoelectric multiferroic behavior is extremely challenging because the constituent properties - ferroelectricity and (ferro)magnetism - are contra-indicated. This is because the constituent parts of extended oxide phases which break the inversion symmetry of the material and lead to ferroelectric behaviour (d0 transition metal cations or ns2 post-transition metal cations), are necessarily diamagnetic and thus make no contribution to the magnetic behaviour of the material.
Magnetic behavior can be introduced into ferroelectric materials by adopting a ‘composite’ strategy which makes use of a combination of paramagnetic cations and diamagnetic symmetry-breaking cations. For example by locating non-spherical, ns2 post transition-metal cations on the 12-coordinate A-sites of an ABO3 perovskite lattice to break inversion symmetry, and paramagnetic transition-metal cations on the 6-coordinate B-sites to introduce magnetism, both prerequisites for magnetoelectric behavior can be fulfilled. Many materials of this type have been prepared, such as BiFeO3 or PbMnO3, for example.
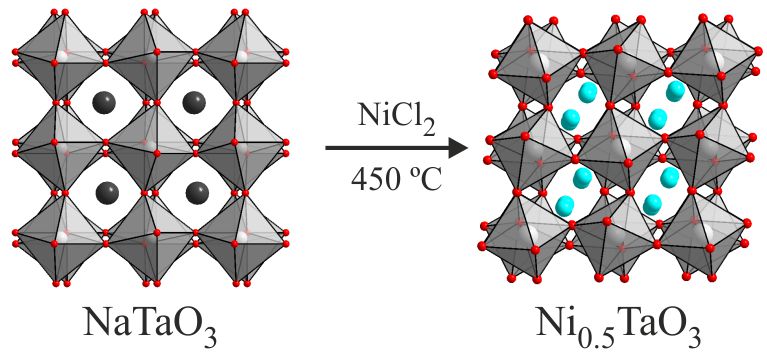
Cation exchange reactions let us prepare the alternative composite materials, with symmetry breaking cations on the B-site and magnetic cations of the A-site of a perovskite lattice. If a 10:1 molar ratio of NiCl2:NaTaO3 is heated at 450 °C for 4 weeks a nickel-for-sodium cation exchange occurs converting NaTaO3 into Ni0.5TaO3 - to our knowledge the first topochemical cation exchange of a 3D perovskite phase. |
 |
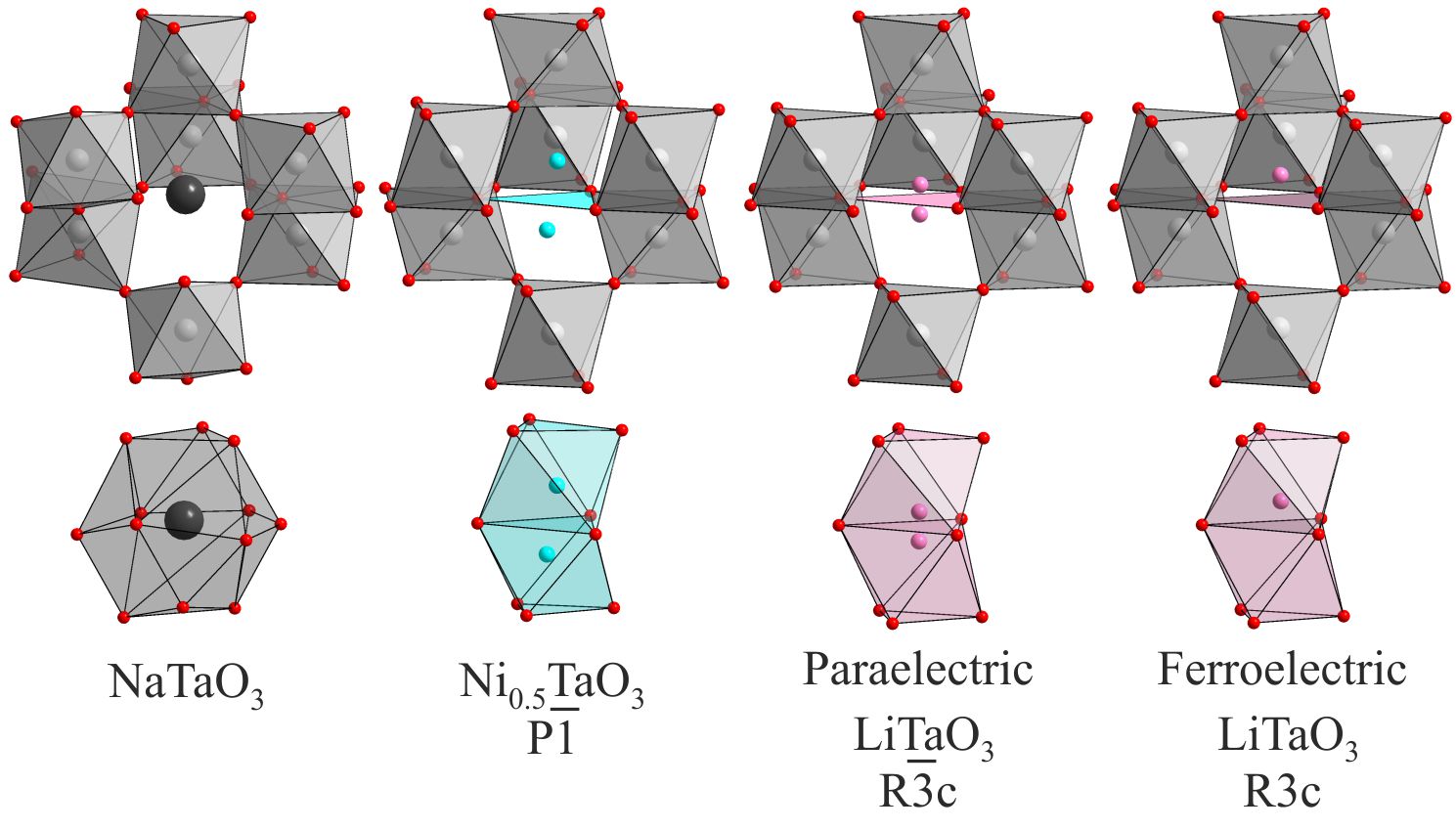 |
Substitution of Na+ cations with much smaller Ni2+ cations leads to a large distortion to the framework of apex-linked TaO6 octahedra, converting the 12-coordinate A-cation site in NaTaO3 into two 6-coordinate sites in the structure of Ni0.5TaO3. The resulting structure is analogous to the paraelectric form of LiTaO3. Unfortunately, unlike LiTaO3, Ni0.5TaO3 does not undergo a transition to a non-centrosymmetric ferroelectric material, remaining paraelectric at all measured temperatures.
Inorganic Chemistry, 53 (2014) 8020. |
Back to Topochemical Synthesis
|

