
 |
| Home | Research | Publications | People | Joining the Group | Teaching |
|
Cation insertion reactions allow the topochemical reduction of extended transition metal oxide phases. A key requirement of this type of reaction is that the host lattice contains an oxidizable cation and a suitable location to accommodate the inserted cation. This second requirement is particularly onerous (the majority of metal-oxide lattices are dense so cannot accommodate additional cations) and limits the widespread applicability of this approach. |
|
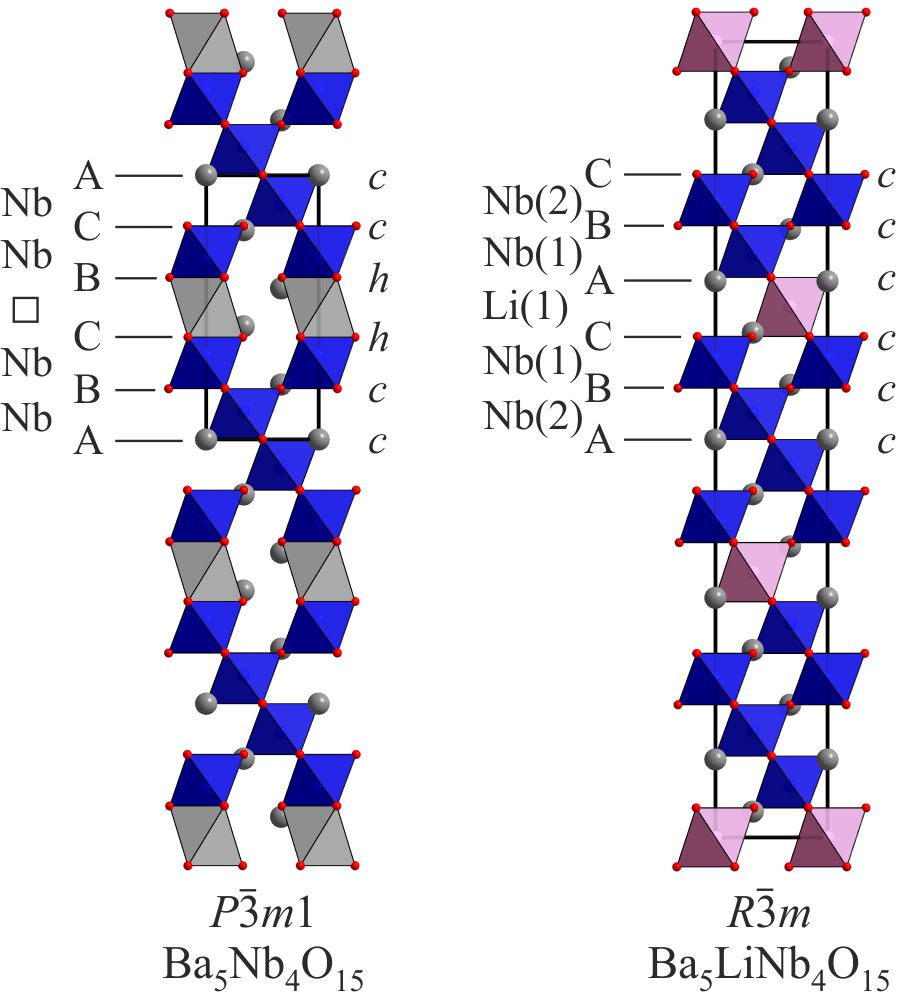
Lithium insertion into cation-deficient perovskite phases Phases which adopt cation-deficient perovskite structures are rare. However when the B-cation is an early transition metals in its group oxidation state (Ti4+, Nb5+, Ta5+, W6+) phases with AnBn-1O3n cation-deficient perovskite structures can be formed, particularly when the A-cation is barium. The B-cation ‘deficiency’ in these phases is accommodated structurally by sheets of vacant octahedral coordination sites (marked in grey in the figure) which can accommodate intercalated cations. Reaction of Ba5Nb4O15 with LiH at 400 ºC leads to the rapid intercalation of lithium and the formation of Ba5LiNb4O15 and the concomitant reduction of niobium. During lithium insertion into Ba5Nb4O15 the ccchh stacking sequence of the host lattice is converted into an entirely cubic stacking sequence, while the B-cation vacancy order of the phases is faithfully converted into Li – Nb order in the lithiated product. The lithiated product exhibits semiconducting behaviour with a temperature dependence consistent with the variable-range-hopping of a small polaron conductor. Dalton Transactions, 44 (2015) 10636. |
 |