|
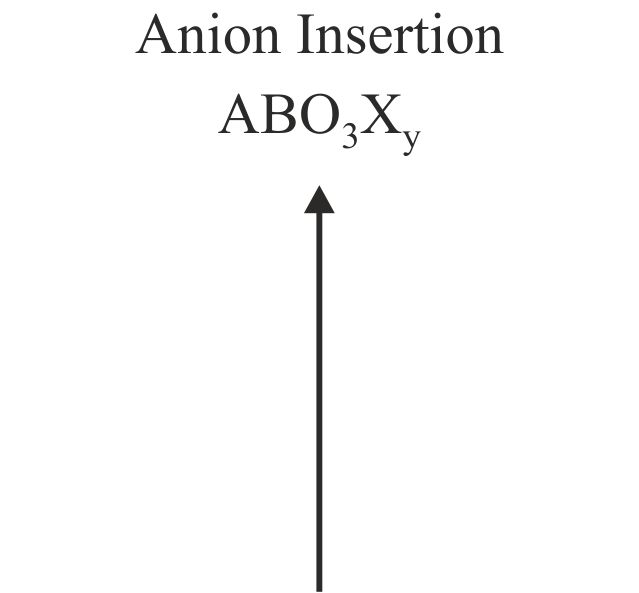
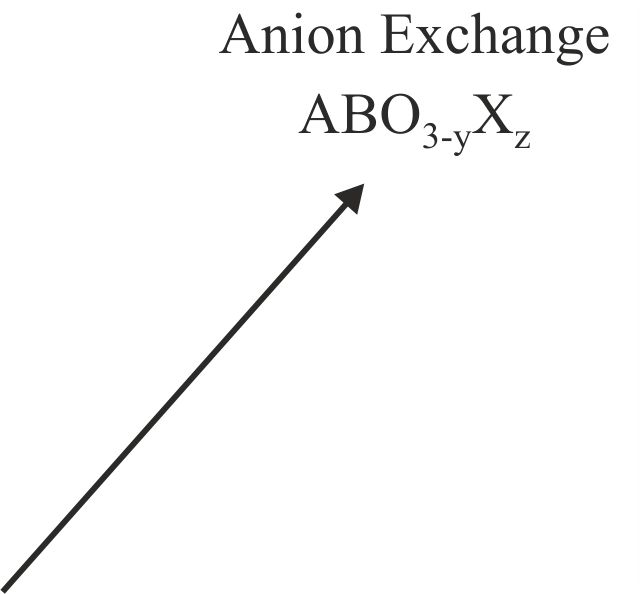
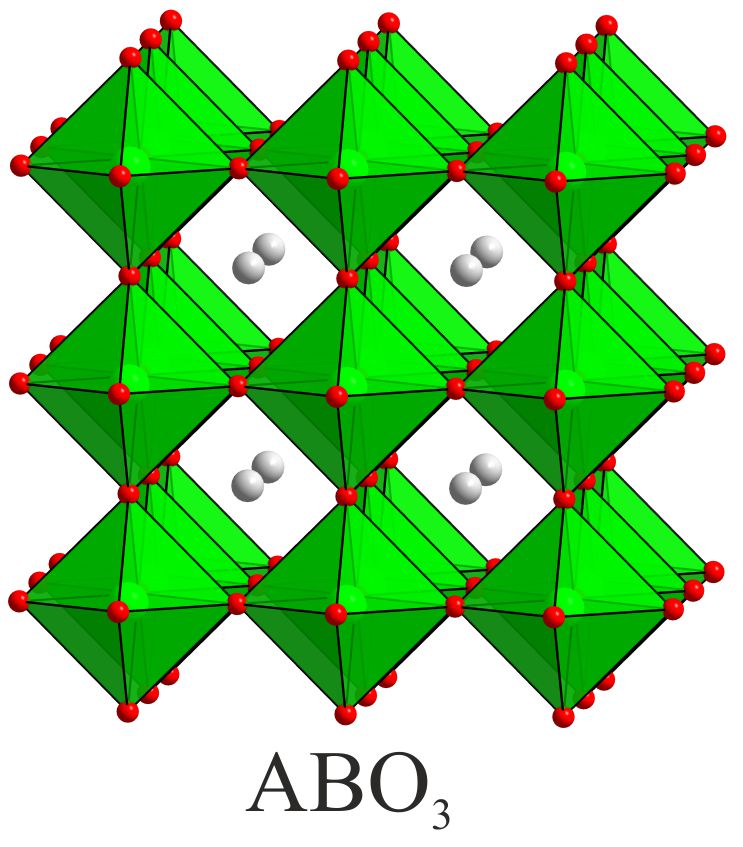
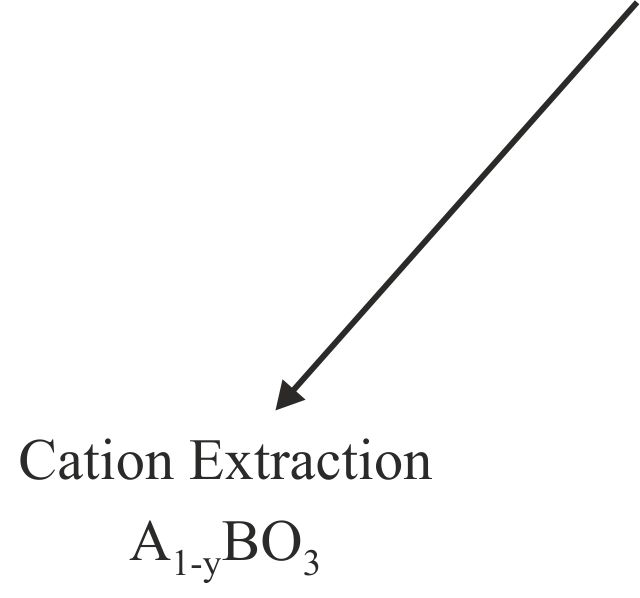
Solid-state reactions performed at low-temperature can allow the synthesis of novel phases, not preparable by conventional high-temperature chemistry, by utilizing the differences in the rates of solid diffusion of the different ions present in the system. For example, in certain complex oxides particular classes of ion are observed to be significantly more mobile, at a given temperature, than the remainder of the host phase in which they reside. As a result these highly mobile species can be inserted into, or removed from, host phases under conditions in which the remaining constituents of the host lattice are effectively immobile. The resulting chemical transformations are described as ‘topochemical’ because they conserve the basic topology of the parent complex oxide phase. The product phases thus formed are generally metastable and synthetically inaccessible by conventional high-temperature synthesis routes.
A more detailed description of topochemical synthesis techniques and other 'soft' chemistry approaches to the synthesis of solids can be found in a recent book chapter: Soft Chemical Synthesis of Oxides by M. A. Hayward, Comprehensive Inorganic Chemistry II, Elsevier (Oxford), Volume 2, pages 417 - 453, ISBN 9780080977744 (Download)
Utilizing these synthetic approachs we seek to prepare new materials with complex, correlated electronic and magnetic behaviour by exploring the influence of unusual combinations of transition metal oxidation state and coordination geometry on physical behaviour.
Click below to see recent work from the group which exploits these transformations |